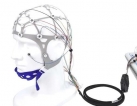
Saddle Stimulating Electrode-Conductive Paste or Saline Wate
China, Shanghai City, Shanghai - view exact locationCategory
Product Information
Order volume from: On request
Delivery terms: Pickup
Description
The EarlyTect Colon Cancer Test is a highly reliable and accurate product that has been approved by the Korea Ministry of Food and Drug Safety. It has undergone rigorous clinical trials at renowned medical centers such as Yonsei Medical Center, Severance Hospital, and Severance Check-up Center.
This test is specifically designed for individuals between the ages of 30 to 80 who are looking for a reliable screening method for colorectal cancer. The clinical trials have shown that the test has a remarkable 90% sensitivity and specificity in diagnosing colorectal cancer.
The EarlyTect Colon Cancer Test utilizes a qualitative real-time PCR technology to measure the level of methylated Syndecan-2, which is a biomarker directly associated with precancerous lesions of colorectal cancer. By analyzing the stool DNA, this test is able to accurately diagnose the presence of colorectal cancer.
It is important to note that this test is not intended to confirm the presence of colorectal cancer. However, if a subject tests positive, it suggests the possibility of the presence of colorectal cancer or its precancerous lesions.
The product meets the following standards:
- Cancer Type: Colorectal Cancer
- Subject: Asymptomatic individuals seeking colorectal cancer screening
- Biomarker: SDC2 me
The price and transaction conditions for the EarlyTect Colon Cancer Test are negotiable. Please feel free to request a quote for further details on the transaction.

 Business
Business