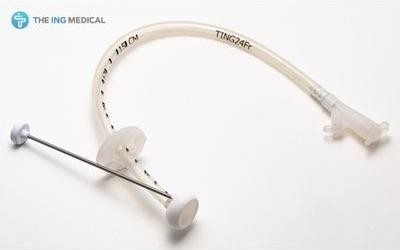
\
Чорногорія, Канто, Токіо - подивитися точне місце розташуванняКатегорія
Інформація про товар
Обсяг замовлення від: За запитом
Умови доставки: Самовивіз
 Бізнесу
Бізнесу
Обсяг замовлення від: За запитом
Умови доставки: Самовивіз
 Медичні бахіли
Ціна за запитом
Туреччина,
Мраморноморський регіон, Стамбул
Медичні бахіли
Ціна за запитом
Туреччина,
Мраморноморський регіон, Стамбул
 Медичний чепчик
Ціна за запитом
Туреччина,
Мраморноморський регіон, Стамбул
Медичний чепчик
Ціна за запитом
Туреччина,
Мраморноморський регіон, Стамбул
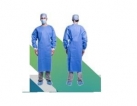 Одноразові медичні халати
Ціна за запитом
Туреччина,
Мраморноморський регіон, Стамбул
Одноразові медичні халати
Ціна за запитом
Туреччина,
Мраморноморський регіон, Стамбул
 Тип IIR Melt Blown 3 PLY Mask
Ціна за запитом
Туреччина,
Мраморноморський регіон, Стамбул
Тип IIR Melt Blown 3 PLY Mask
Ціна за запитом
Туреччина,
Мраморноморський регіон, Стамбул
 Маска проти запотівання
Ціна за запитом
Туреччина,
Мраморноморський регіон, Стамбул
Маска проти запотівання
Ціна за запитом
Туреччина,
Мраморноморський регіон, Стамбул
 Одноразовий індивідуальний захисний одяг
Ціна за запитом
Туреччина,
Мраморноморський регіон, Стамбул
Одноразовий індивідуальний захисний одяг
Ціна за запитом
Туреччина,
Мраморноморський регіон, Стамбул
 \
Ціна за запитом
Чорногорія,
Канто, Токіо
\
Ціна за запитом
Чорногорія,
Канто, Токіо
 Системи зарядки для будь-якої прикладної області
Ціна за запитом
Чорногорія,
Канто, Токіо
Системи зарядки для будь-якої прикладної області
Ціна за запитом
Чорногорія,
Канто, Токіо
 9339
Ціна за запитом
Чорногорія,
Канто, Токіо
9339
Ціна за запитом
Чорногорія,
Канто, Токіо
 Eрлітект® рак кишечника
Ціна за запитом
Чорногорія,
Канто, Токіо
Eрлітект® рак кишечника
Ціна за запитом
Чорногорія,
Канто, Токіо
Цей сайт використовує cookie файли і інші ідентифікатори відвідувачів для комфортної роботи кожного користувача. Якщо, прочитавши це повідомлення, ви залишитеся на нашому сайті, це означає, що ви не заперечує проти використання цих технологій. Дізнатися більше