USCOM LTD.: каталог та список продуктів USCOM LTD. на europages. Угорщина, 1-3
Угорщина, Центральна Угорщина , Будапешт - подивитися точне місце розташуванняКатегорія
Інформація про товар
Обсяг замовлення від: За запитом
Умови доставки: Самовивіз
Основна інформація
- Доступно на:
- English
- Український
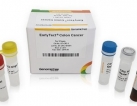
Introducing the EarlyTect Colon Cancer Test, an advanced screening tool approved by the Korea Ministry of Food and Drug Safety. This groundbreaking product has undergone extensive clinical trials at reputable medical centers, including Yonsei Medical Center, Severance Hospital, and Severance Check-up Center.
Designed for both men and women aged 30 to 80, the EarlyTect Colon Cancer Test utilizes fecal DNA analysis to accurately diagnose colorectal cancer. With a remarkable 90% sensitivity and specificity rate, this qualitative real-time PCR test measures the presence of methylated Syndecan-2, a biomarker closely associated with precancerous lesions of colorectal cancer.
It is important to note that the EarlyTect Colon Cancer Test is not intended to provide a confirmation of colorectal cancer. However, a positive result may indicate the presence of colorectal cancer or precancerous lesions, prompting further investigation and necessary follow-up procedures.
Product Standard:
- Cancer Type: Colorectal Cancer
- Subject: Asymptomatic individuals seeking colorectal cancer screening
- Biomarker: Methylated Syndecan-2
For pricing and transaction details, we offer negotiable terms. Please feel free to request a quote to initiate the transaction process.

 Бізнесу
Бізнесу