Fio de Eletrodo de Taça de Ouro EEG Reutilizável
China, Cidade de Shanghai, Xangai - veja a localização exataInformações do produto
Volume de pedidos de: A pedido
Termos de entrega: Escolher
Volume de pedidos de: A pedido
Termos de entrega: Escolher
 Capacete EEG - para Eletrodos em Copo
Preço em pedido
China,
Cidade de Shanghai, Xangai
Capacete EEG - para Eletrodos em Copo
Preço em pedido
China,
Cidade de Shanghai, Xangai
 Cabo Snap-Fio e Eletrodo Tipo Botão
Preço em pedido
China,
Cidade de Shanghai, Xangai
Cabo Snap-Fio e Eletrodo Tipo Botão
Preço em pedido
China,
Cidade de Shanghai, Xangai
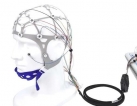 1412
Preço em pedido
China,
Cidade de Shanghai, Xangai
1412
Preço em pedido
China,
Cidade de Shanghai, Xangai
 EEG Cap - para Eletrodos de Copa
Preço em pedido
China,
Cidade de Shanghai, Xangai
EEG Cap - para Eletrodos de Copa
Preço em pedido
China,
Cidade de Shanghai, Xangai
 5169
Preço em pedido
China,
Cidade de Shanghai, Xangai
5169
Preço em pedido
China,
Cidade de Shanghai, Xangai
 Eletrodo Estimulante de Sela - Pasta Condutora ou Água Salina
Preço em pedido
China,
Cidade de Shanghai, Xangai
Eletrodo Estimulante de Sela - Pasta Condutora ou Água Salina
Preço em pedido
China,
Cidade de Shanghai, Xangai
 0269
Preço em pedido
China,
Cidade de Shanghai, Xangai
0269
Preço em pedido
China,
Cidade de Shanghai, Xangai
 EMG 5 Pin-Connector 1 Para 2 Eletrodo de Copa Dourada
Preço em pedido
China,
Cidade de Shanghai, Xangai
EMG 5 Pin-Connector 1 Para 2 Eletrodo de Copa Dourada
Preço em pedido
China,
Cidade de Shanghai, Xangai
 Anel de Eletrodo
Preço em pedido
China,
Cidade de Shanghai, Xangai
Anel de Eletrodo
Preço em pedido
China,
Cidade de Shanghai, Xangai
 0882
Preço em pedido
China,
Cidade de Shanghai, Xangai
0882
Preço em pedido
China,
Cidade de Shanghai, Xangai
 Pacotes de bateria recarregáveis específicos para clientes.
Preço em pedido
China,
Cidade de Shanghai, Xangai
Pacotes de bateria recarregáveis específicos para clientes.
Preço em pedido
China,
Cidade de Shanghai, Xangai
 5601
Preço em pedido
China,
Cidade de Shanghai, Xangai
5601
Preço em pedido
China,
Cidade de Shanghai, Xangai