EarlyTect® Colon Cancer
Hungary, Central Hungary, Budapest - view exact locationCategory
Product Information
Order volume from: On request
Delivery terms: Pickup
Description
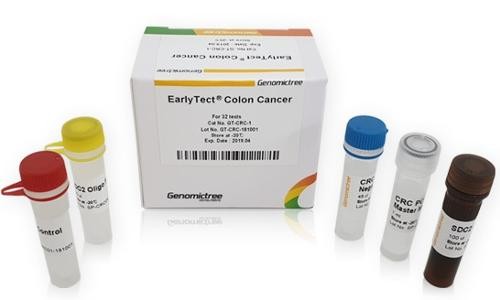
Introducing EarlyTect Colon Cancer Test, a groundbreaking product in the field of colorectal cancer screening. This test has been approved by the Korea Ministry of Food and Drug Safety following extensive clinical trials conducted at prestigious institutions such as Yonsei Medical Center, Severance Hospital, and Severance Check-up Center.
What sets EarlyTect Colon Cancer Test apart is its utilization of fecal DNA analysis to detect colorectal cancer (CRC) with unprecedented accuracy. Through these clinical trials, it has been demonstrated that the test has a remarkable 90% sensitivity and specificity in diagnosing CRC. By measuring methylated Syndecan-2, a biomarker associated with precancerous lesions of CRC, in stool DNA, EarlyTect Colon Cancer Test provides a reliable and qualitative real-time PCR test for the early detection of colorectal cancer.
It is important to note that EarlyTect Colon Cancer Test is not designed to confirm the presence of colorectal cancer. However, a positive result from the test indicates the potential existence of CRC or its precancerous lesions, prompting further investigation and appropriate medical consultation.
Product Standard:
- Cancer Type: Colorectal Cancer
- Target Subject: Asymptomatic individuals undergoing colorectal cancer screening
- Biomarker: SDC2 methylation
We understand that pricing and transaction conditions will vary depending on various factors. Therefore, we invite you to request a quote for a personalized offer that meets your specific requirements.
In conclusion, EarlyTect Colon Cancer Test is a cutting-edge solution in the realm of colorectal cancer screening. With its highly accurate results and the potential to detect the disease at an early stage, this test offers hope in improving patient outcomes and reducing the impact of colorectal cancer.


 Business
Business