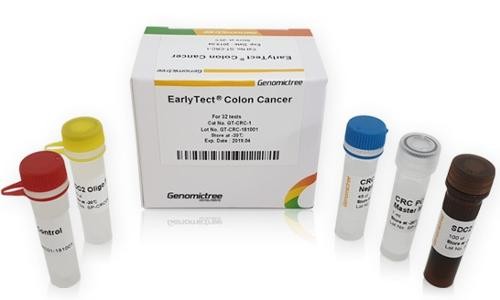
سرطان القولون المبكر (EarlyTect®)
الجبل الأسود, كانتو, طوكيو - انظر الموقع الدقيقالفئة
معلومات المنتج
حجم الطلب من: تحت الطلب
شروط التوصيل: يلتقط
السعر عند الطلب


 اعمال
اعمال